Tiny particles that are 10,000x minute than the breadth of a hair, nanodiamonds, can avoid lithium batteries from bursting into fire, making your laptops, smartphones, and other mobile gadgets more secure to use, researchers claimed.
Scientists, comprising those in the U.S. at Drexel University, designed a formula that can convert electrolyte solution, which is an important element for most of the batteries, into a protection against the chemical procedure that results in disasters related to battery. The group aimed their work on turning lithium plating more consistent and lithium anodes more steady by including nanodiamonds in a battery to the electrolyte solution.
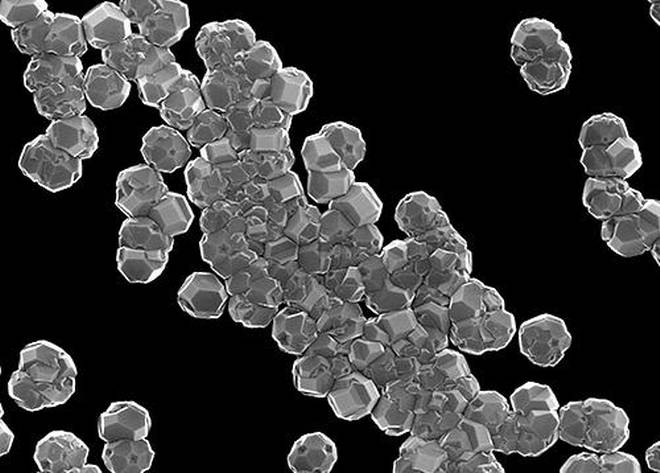
Nanodiamonds have been utilized in the electroplating segment for some time as an approach of turning metal coatings more consistent. While they are much cheaper and smaller than the diamonds employed in jewellery, nanodiamonds still keep hold of the regular shape and structure of their expensive progenitors. When they are put down and they naturally glide together to make a flat surface.
Scientists discovered that lithium ions can effortlessly connect to nanodiamonds, so when they are electroplating the electrode, it is in the similar orderly way as the nanodiamond particles to which they are connected. Mixing nanodiamonds into the solution of electrolyte for a lithium ion battery decreased the dendrite formation to nil via 100 cycles of charge-discharge, scientists claimed to the media.
This detection is just the start of a procedure that might ultimately view electrolyte additives, such as nanodiamonds, extensively utilized to make secure lithium batteries with an elevated density of energy, said researchers. Original results already display steady charge-discharge cycling for almost 200 Hours, which is sufficiently long for use in some military or industrial applications, but not almost sufficient for batteries utilized in cell phones or laptops, they added.
“It is potentially game-turning, but it is hard to be 100% sure that dendrites will never develop,” claimed professor at Drexel University, Yury Gogotsi, to the media in an interview. “To guarantee safety, additives to electrolytes (nanodiamonds) require to be mixed with other safety measures, such as utilizing safer electrode materials, non-flammable electrolytes, and stronger separators,” Gogotsi further added.
###